The Brelidze Lab: A Story of Collaboration and Scientific Resilience

Posted in GUMC Stories | Tagged biomedical research, ion channels, pharmacology, physiology, research
(January 9, 2024) — When Tinatin “Tina” Brelidze, PhD, began looking at universities to start a lab following her postdoctoral fellowship at the University of Washington, she sought to join a collaborative environment with a strong student population and vibrant cultural community.

Georgetown University Medical Center fit the bill, and in 2013 she joined the Department of Pharmacology and Physiology.
“It is very important to establish collaborations with other people,” said Brelidze, an associate professor. “This is why Georgetown is wonderful. Many people do different types of research and they are willing to collaborate, and you can enrich your experiments.”
Brelidze studies ion channels, membrane proteins involved in many diseases. At Georgetown, she has collaborated with multiple colleagues on research, including Yuichiro “Justin” Suzuki, PhD, professor of pharmacology and physiology, on the role of ion channels in cardiovascular diseases; Rhonda Dzakpasu, PhD, associate professor of physics, on the role of the ion channels in neural dynamics; and Anton Wellstein, MD, PhD, professor of oncology and pharmacology, on the role of ion channels in cancer.
Ion Channels Explained
Ion channels are cell membrane proteins that act like a door, allowing the passage of ions such as sodium, potassium, chloride and calcium to either enter or leave the cell. This process will negatively or positively affect the cell membrane electrical charge distribution.
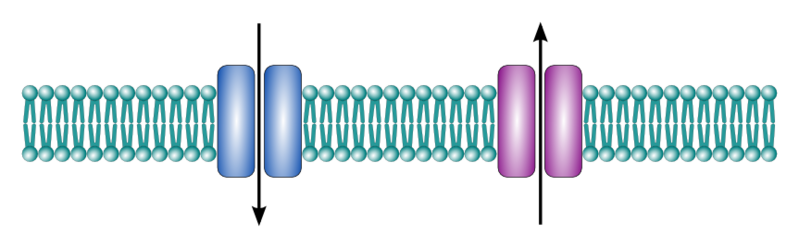
Ion channels can be classified by the “key” required to open them. For example, if an ion channel’s opening mechanism is a change in the cell membrane charge distribution, it is considered a voltage-gated ion channel. Similarly, if a specific molecule (ligand) binding to the ion channel causes it to open or close, it is considered a ligand-gated ion channel.
Brelidze spent her first two years at Georgetown studying the structure of ion channels through X-ray crystallography, a method used to visualize proteins. However, setbacks with her structural studies and the availability of other methods at Georgetown inspired her to pursue another line of ion channel research.
“It is an important skill to realize early on if you are moving in the right direction, and then refocus and regroup and start developing new directions,” Brelidze said. “You have to keep constantly pushing yourself and learning new techniques and new approaches. It is a constant rediscovery and integration of various methods.”
Currently, the Brelidze lab is investigating the ligand-gating properties of the KCNH1 channel, a potassium-ion channel expressed in higher numbers in certain cancers, such as neuroblastoma, breast and lung cancers.
Her lab was the first to show that important regulatory domains of KCNH1 channels can bind small molecule ligands, and that this binding can regulate the function of KCNH1 channels. Considering the increased expression of KCNH1 channels in many cancers, the ligands have a potential to impact tumor cell growth.
Drug Repurposing
Some of the small molecule ligands of KCNH1 channels identified by Brelidze’s lab are FDA-approved medications. Her team is testing these in animal models and cell cultures to see if any of them decrease tumor growth.
“You must be very resilient to succeed in science,” said Tina Brelidze.
Recently, the Brelidze lab discovered FDA-approved medications, including the antidepressant imipramine and antipsychotic medication chlorpromazine, that inhibit tumor cell growth by stopping the passage of potassium through KCNH1 channels.
The common practice of drug repurposing in pharmacological research has multiple benefits, including increasing the speed of discovery for novel treatments — since these drugs have passed multiple necessary safety screenings — and decreasing the cost of developing an entirely new therapeutic compound.
The estimated research and design cost for anticancer medications is the highest compared to other medications — between $944 million and $4.54 billion — further supporting the necessity of assessing already available drug treatments and their potential role in cancer treatment.
“You hope for the best, but you need to be patient,” Brelidze said. “You must be very resilient to succeed in science.”
by Carleigh Turner
Carleigh Turner is a second-year PhD student in the Pharmacology and Physiology Program and a trainee in the Pharmacological Sciences Training Program.
