Unique “Home Built” Device Provides Fast Disease Analysis in Kidneys Affected by Diabetes
Posted in News Release | Tagged biomedical research, diabetes research, imaging, kidney research, microscopy, pathology
Media Contact
Karen Teber
km463@georgetown.edu
WASHINGTON (May 28, 2020) — The amount of scarring in damaged kidneys as a result of diabetes or acute injury is a key factor in determining treatment. But it has not been possible, using traditional techniques, to quickly and accurately assess how widespread this kind of wounding extends within the organ. Now, however, a physicist and chemist at Georgetown University Medical Center has shown that a microscope he began developing with colleagues at University of California, Irvine can provide an immediate answer.
His findings, published in the journal Kidney International, suggest that, given further successful testing of this device, it could be adopted in an operating suite using biopsies, usually taken with a needle, from a patient’s kidney. These biopsies, which don’t need to be stained, will score the degree of tubulointerstitial fibrosis — progressive scarring due to a failed wound-healing process of kidney tissue after chronic, sustained injury. This score can then be combined with results from traditional pathology to help physicians assess long-term prognosis.
While this kind of approach was developed for cancer prognosis, this study represents “to our knowledge, the first expansion of this type of test to understand human kidney disease and to specifically to characterize disease states,” says the study’s lead investigator, Suman Ranjit, PhD, assistant professor in Georgetown’s Department of Biochemistry and Molecular & Cellular Biology.
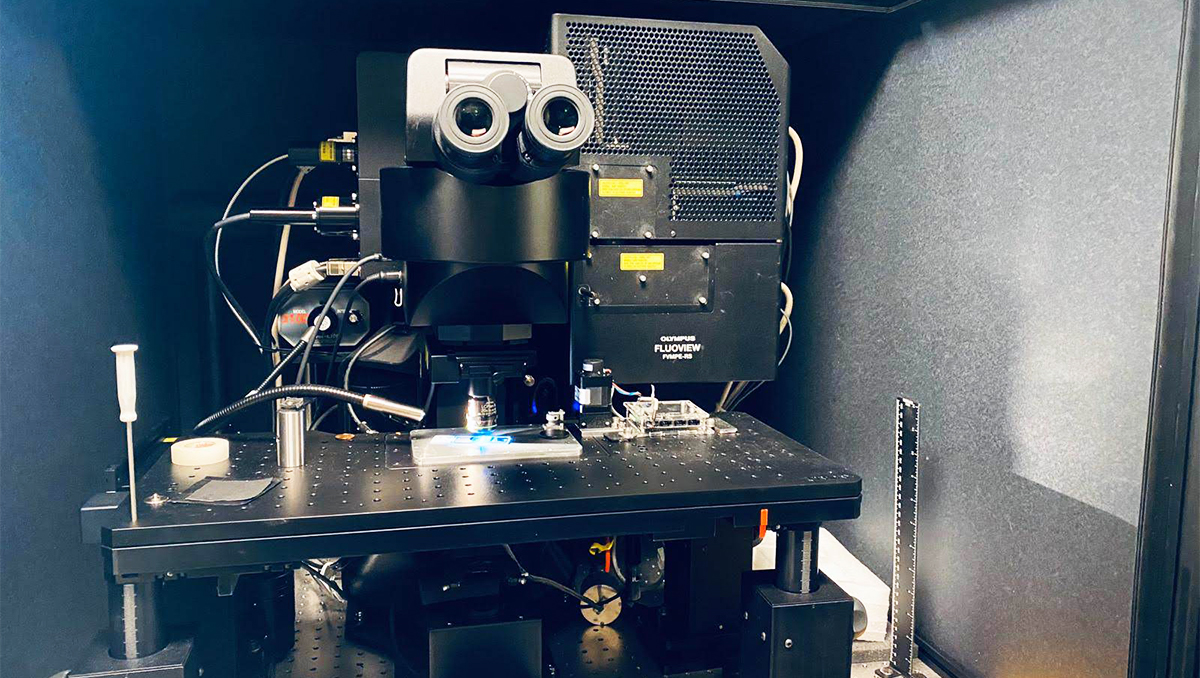
The advanced microscope, called DIVER, uses phasor approach to fluorescence lifetime imaging (FLIM). Simply said, the devices work together to examine the type of molecules that are in an image of the tissue sample captured by the microscope. It uses endogenous fluorescence emitted naturally by the biomolecules, measuring the time it takes for different molecules to stay in the excited state (fluorescence lifetime). The results are pseudo-color mapped, with each color representing specific types and degrees of molecular content that reveal changes in structure and biology of the organ that link to disease severity.
“Using this method, numerous biopsies from a kidney can be examined quickly. The process is automated, eliminating operator bias,” says Ranjit. In contrast, traditional biopsies — often an hourslong process of staining and pathological examination — happens outside of an operating room.
This study examined frozen human kidney biopsy tissues from patients with diabetes, obtained from the University of Chicago. Researchers found that the new method closely replicated findings obtained by pathology analyses.
Ranjit started to work on this clinical project using what he calls his “home built” device while a postdoctoral scholar at UC Irvine’s Laboratory for Fluorescence Dynamics, but completed it at Georgetown. He now has applied for federal grants that will help him “shrink” this idea into a small handheld device that could be used in operating rooms, and to further improve automation and imaging speed.
When the device is perfected, Ranjit says it may be possible to use it for assessing the disease state in many organs, a process that now depend on cumbersome pathological analysis.
“When widely used, this imaging technique will enable physicians to detect early fibrosis, or scarring, in tissues as well as determine the health of kidney, liver and other tissues that are being considered for transplantation,” says co-author Moshe Levi, MD, Interim Dean for Research at GUMC and professor of biochemistry and molecular & cellular biology.
In addition to Ranjit and Levi, co-authors include Kammi Henriksen, MD, PhD (co-lead author), University of Chicago; Alexander Dvornikov, PhD, University of Califorina-Irvine; Marco Delsante, MD, University of Parma, Italy; Avi Rosenberg, MD, PhD, Johns Hopkins University; and Enrico Gratton, PhD, UC Irvine.
Gratton holds a patent related to the microscope described. No other competing financial interests are reported.
Funding for this research was provide by National Institutes of Health grants (P50 GM076516, P41GM103540, 1R01DK098336) and Veterans Affairs Medical Research (1I01BX001954).
