Georgetown University Launches Clinical Trial for Lewy Body Dementia
Posted in News Release | Tagged biomedical research, brain, Lewy body dementia, mind, neurology
MEDIA CONTACT:
(for members of the press only)
Karen Teber
km463@georgetown.edu
PATIENT INFORMATION:
Joy Arellano
mja6@gunet.georgetown.edu
WASHINGTON (May 30, 2019) — Georgetown University Medical Center announces the launch of the only known therapeutic (disease modifying) clinical trial for Lewy body dementia, a neurological disorder that affects a million people in the United States for which there are no approved medications that modify the disease.
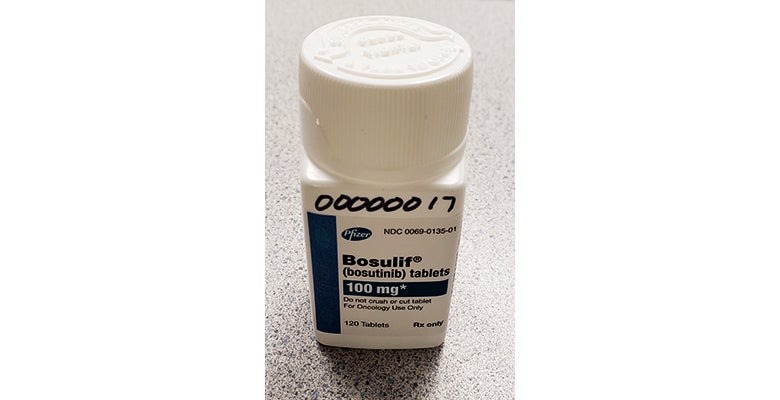
The researchers are studying bosutinib (Bosulif®) — approved by the U.S. Food and Drug Administration (FDA) to treat a form of leukemia, but not approved for other uses.
The repurposing of this cancer drug for neurologic disorders is based on research from the Laboratory of Dementia and Parkinsonism at Georgetown University Medical Center, a Lewy Body Dementia Association “Research Center of Excellence.”
The clinical trial’s principal investigator, Fernando Pagan, MD, says a treatment for Lewy body dementia, the second most common cause of dementia, is desperately needed.
“Lewy body dementia is often confused with Alzheimer’s or Parkinson’s disease,” says Pagan, medical director of Georgetown’s Translational Neurotherapeutics Program (TNP) and director of the Movement Disorders Clinic at MedStar Georgetown University Hospital. “People with Lewy body dementia experience various symptoms, including problems with cognition or memory, behavior and mood, but people with this disorder also have movement problems like those seen with Parkinson’s disease.”
“The first clinical step in studying bosutinib in Lewy body dementia is to carefully examine if the drug is safe and if it is tolerable, and we’ll do that in this trial,” Pagan says. “We’ll also look to see if they can change the levels of the abnormal proteins and dopamine in the blood and spinal fluid.”
Thirty (30) patients with Lewy body dementia will be enrolled for the bosutinib study through MedStar Georgetown, Georgetown’s clinical partner.
The study is a phase II, randomized double blind, placebo controlled clinical trial, meaning study participants will be randomly selected by a computer to receive the active drug (bosutinib) or an inactive pill called a placebo. Neither the patient nor the health care providers will know whether the study participant is taking the active drug or placebo (double blinded). Participants must have a study partner to accompany them to clinic visits.
Alpha-synuclein accumulation can cause the loss of dopamine, which controls movement, emotions, attention and other cognitive functions. Other proteins, beta-amyloid and tau, are also thought to contribute to the disease.
Animal studies have shown that bosutinib can lower the levels of alpha-synuclein, tau and beta-amyloid; reverse the loss of dopamine; and reduce inflammation in the brain. These animal studies have also shown that bosutinib may be able to decrease inflammation and reverse dopamine loss at lower doses than what is used for leukemia.
Bosutinib is the second investigational drug developed by the TNP to be validated in animal studies and advance to clinical trials.
“We have been studying bosutinib for almost a decade as a potential therapy to modify neurodegenerative pathologies, including Lewy body dementia,” says Charbel Moussa, MBBS, PhD, clinical and scientific research director of the TNP and director of the Laboratory of Dementia and Parkinsonism.
“In animal models, bosutinib ameliorates neurodegenerative pathologies and behavior at much lower doses than the cancer dose, providing strong feasibility to study this drug in individuals with LBD,” adds Moussa.
The clinical trial is being funded by an Alzheimer’s Association Part the Cloud grant (#PTC19-604325) received by Moussa.
Patients seeking information about this and other dementia or movement disorders research should contact Joy Arellano at mja6@gunet.georgetown.edu. Additional information can be found at clinicaltrials.gov.
The FDA has approved an “investigation new drug” (IND) application submitted by Georgetown to study bosutinib.
Georgetown University has a granted patent in Australia and pending patent applications in U.S. and other foreign jurisdictions on the use of bosutinib to treat alpha-synucleopathies. Moussa, a co-investigator on the study, is named as the inventor on the patent.
About Georgetown University Medical Center
Georgetown University Medical Center (GUMC) is an internationally recognized academic health and science center with a four-part mission of research, teaching, service and patient care (through MedStar Health). GUMC’s mission is carried out with a strong emphasis on public service and a dedication to the Catholic, Jesuit principle of cura personalis — or “care of the whole person.” The Medical Center includes the School of Medicine and the School of Nursing & Health Studies, both nationally ranked; Georgetown Lombardi Comprehensive Cancer Center, designated as a comprehensive cancer center by the National Cancer Institute; and the Biomedical Graduate Research Organization, which accounts for the majority of externally funded research at GUMC including a Clinical and Translational Science Award from the National Institutes of Health. Connect with GUMC on Facebook (Facebook.com/GUMCUpdate) and Twitter (@gumedcenter).
